CT Models Predict COPD and Smoking-Related Morbidity in Cigarette Smokers
Tuesday, Nov. 29, 2016
Biomarkers extracted from inspiratory CT scans during lung cancer screenings reveal measures for emphysema and airway obstruction useful in predicting chronic obstructive pulmonary disease (COPD) and smoking-related morbidity in cigarette smokers, according to research presented Monday.

Jean-Paul Charbonnier, MS, PhD
Researcher Jean-Paul Charbonnier, MS, PhD candidate at Radboud University in the Netherlands, discussed the results of a study using quantitative CT (QCT) aimed at quantifying features related to COPD and smoking-related morbidity. The models developed may be used in clinical practice to more quickly provide insights into patients' health and, in the case of lung cancer screenings, detect more than just pulmonary nodules.
Setting the framework
Charbonnier and his colleagues examined data from 1,544 subjects participating in the first phase of the COPDGene® study, one of the largest studies to investigate causal genetic factors of COPD. They used clinical and functional characteristics collected from subjects during physical examinations. Additionally, they performed image analysis on available inspiratory and expiratory CT scans to quantify emphysema, airway dimensions and air trapping.
Based on QCT, COPD was defined by a ratio of forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) <0.7. Smoking-related morbidity was defined as FEV1/FEVC <0.7 with either a St. George's Respiratory Questionnaire score greater than or equal to 25 or an exacerbation frequency greater than or equal to two.
During the extraction of quantitative information, the researchers faced some challenges in comparing subjects' lungs, typically because of differences in anatomy or scanner parameters. "Variations can be difficult for automated algorithms that perform the CT quantification," Charbonnier said. "Visual assessments are sometimes needed and take a substantial amount of time and effort."
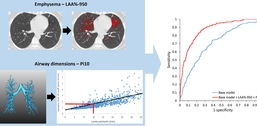
Prediction of smoking-related morbidity (defined as having COPD with more than 1 exacerbation per year or a St. George's Respiratory Questionnaire score ≥ 25) using quantitative CT (QCT) derived measures from inspiratory CT. The base model consisted of age, gender, BMI, pack years, smoking status and total lung capacity. The QCT model additionally included emphysema (A) (LAA percent -950) and airway dimensions (B) (Pi10). The performance of the prediction models was assessed on a validation data set (585 subjects with and 212 subjects without smoking-related morbidity), with an area under the ROC curve of 0.72 for the base model (blue ROC curve), and 0.88 for the QCT model (red ROC curve) (C).
Emphysema was defined as the percentage of low-attenuation areas <-950HU (LAA percent -950). Airway wall abnormalities were also defined on inspiratory CT using measurements taken from multiple cross-sectional lumen perimeters and airway wall areas. Using linear regression, the measurements were represented as the square root of wall area of an airway with a perimeter of 10 mm (Pi10). Air trapping was defined on expiratory CT as the percentage of low-attenuation areas <-856HU (LAA percent -856).
Developing prediction models
The researchers fitted six logistic regression models for the prediction of both COPD and smoking-related morbidity using a random subset of 747 subjects. The models were validated on a separate subset of 797 subjects using the area under the receiver operating curve.
Model 1 included age, gender, BMI, smoking status, total lung capacity and pack years — a calculation multiplying the number of packs of cigarettes smoked per day by the number of years a person has smoked. Models 2 through 6 additionally included emphysema (model 2), air trapping (model 3), airway dimensions (model 4), emphysema and airway dimensions (model 5) and emphysema, air trapping and airway dimensions (model 6).
Findings revealed that CT-quantified emphysema, air trapping and thickening of the airway walls are all predictors of COPD and smoking related morbidity. They were also independent predictors of smoking-related morbidity in all except for LAA percent -950 in model 5. Because emphysema and airway wall thickening can be detected on inspiratory CT, Charbonnier said the conditions could be detected during standard lung cancer screenings.
Employing computer-based algorithms, the team assessed the large amount of data and provided a level of disease quantification that, according to Charbonnier, would otherwise be difficult or even impossible for human observers. He said, "Automatic and semi-automatic methods to analyze medical images are potentially important tools to assess image information more accurately and effectively and help medical experts make a faster decision with more confidence."
Charbonnier noted that the additional time required to review the QCT results happens offline and does not add time to the patient's screening experience. "We live in an age in which technology plays an important part in our daily life," he said, "and advances in medical imaging pave the way for better and faster diagnosis of many different diseases."



 Home
Home Program
Program Exhibitors
Exhibitors My Meeting
My Meeting
 Virtual
Virtual Digital Posters
Digital Posters Case of Day
Case of Day

